
OPENING QUESTION:
How does violet light differ from red light in terms of the physical characteristics of the EM waves?
How does violet light differ from red light in terms of the physical characteristics of the light particles?
═══════════════════════════
LEARNING TARGET: I will review various characteristics of the EM spectrum during today's class
WORDS O' THE DAY:
- photon (a wave AND a particle of light)
- electromagnetic waves
- energy levels
- excited (electron) = ("electrons 'jolted' into a higher energy level")
- ground state (electron) = ("electrons existing on the lowest energy level")
CALENDAR:
WORK O' THE DAY:
EQ Waves Test Returns -- there were a lot of distractions in the previous couple of weeks, many of us rose above those and crushed the test, an equal number of us, well let's just we didn't.
═══════════════════════════
I'll pass this graphic out for you. Please take a few moments to familiarize yourself with the information (generally) shown there.
Please note in particular that the graph isn't even close to scale-- that is key!
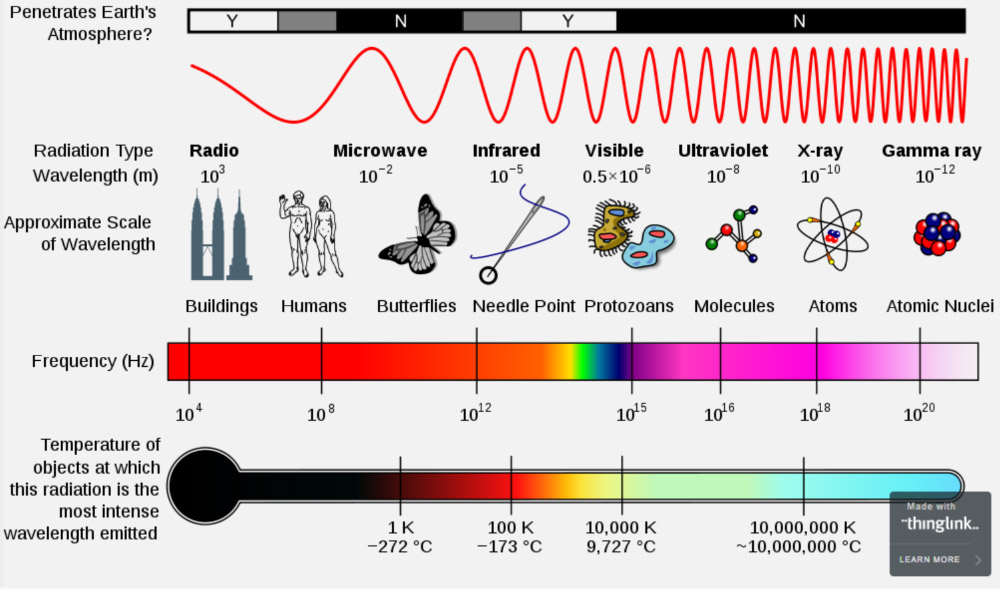
Which of those types of EM radiation/light is *by far* the largest in terms of both wavelength but also width of EM spectrum in which is resides?
Let's discuss!
YIKES:

What can we take away from all of that? Please discuss!
Let's revisit a bit of work from yesterday:
Take a look at the following chart and determine the type of light present for each wavelength of light as it is created by each individual 'step down' for the Lyman Series and Paschen Series shown below:

Here's an interesting question for you-- there are 4 (count 'em FOUR) paths shown in the Balmer Series but 5 spectral lines for hydrogen. Why might that be? Please discuss.

Now please break out your basic photon drawing activity from last time.
We talked about how the 'primary' colors of light are Red Green and Blue.
Let's go HERE to get a visual on how that works-- remember, you are fighting many years of incorrect knowledge/association: we learned that blue and yellow make green way back in 1st grade! Unfortunately that's color pigments in paint and is NOT the same as light waves.
With that in mind work to find an online color mixing program and get a better idea of what color each band and on that worksheet would appear to our eyes
(Oh and there is a VERY big caveat/condition for that to be true-- what might that be?)
Let's make sure we are clear with that before we continue.
THIS website can be helpful but it does have limitations -- we'll come back to this site later for some other practice but feel free to find other sites too!
Years ago I had a parent who was an IR consultant (he had a VERY cool IR camera) come in and talk to my freshman science class. He made a very interesting comment that all objects in the Universe either
- Emit Light
- Absorb Light
- Reflect Light
NOTE: We won't be talking about how objects transmit light (allow light waves to pass through), since that involves things like refraction which can be kind of convoluted.
With that in mind, why does emission (producing), absorbing (taking) and reflecting (returning) have such a profound impact on how we as humans experience the physical world?
